
49 Balancing Chemical Equations Worksheets [with Answers]
Here are 50 simple balanced chemical equations. 2Fe2O3+3C--->4Fe+3CO2 2)H2SO4+CaCO3--->CaSO4+H2CO3 3) 2H2+02--->2H20. What is the balanced chemical equation for magnesium hydroxide and hydrochloric acid? Medium. View solution > When balancing a chemical equation, why can you change coefficients, but not subscripts?.

50 balanced chemical equations? Brainly.in
The total number of atoms in a compound is the subscript multiplied by the coefficient (e.g., 4H 2 O contains 4 x 2 = 8 atoms of hydrogen and 1 x 4 = 4 atoms of oxygen). Balanced Chemical Equations 6 CO 2 + 6 H 2 O → C 6 H 12 O 6 + 6 O 2 (balanced equation for photosynthesis) 2 AgI + Na 2 S → Ag 2 S + 2 NaI Ba 3 N 2 + 6 H 2 O → 3 Ba (OH) 2 + 2 NH 3
How to Balance Chemical Equations? Best Examples Get Education Bee
Balancing Chemical Equations in Five Easy StepsBalancing chemical equations is a core skill in chemistry. In this video you'll learn the basics for balancin.

How to Balance Chemical Equations 11 Steps (with Pictures)
CHEM 120: Fundamentals of Chemistry 4: Chemical Quantities and Reactions

Balancing Chemical Equations — Overview & Examples Expii
Balance a chemical equation when given the unbalanced equation. Explain the role of the Law of Conservation of Mass in a chemical reaction. Even though chemical compounds are broken up and new compounds are formed during a chemical reaction, atoms in the reactants do not disappear, nor do new atoms appear to form the products.
Balancing Chemical Equations VISTA HEIGHTS 8TH GRADE SCIENCE
Balancing chemical equations 1 Google Classroom Balance the following chemical equation: Mg (OH) 2 + HCl → MgCl 2 + H 2 O Note: All reactants and products require a coefficient of at least one. Stuck? Review related articles/videos or use a hint. Report a problem Do 4 problems

How to balance chemical equations
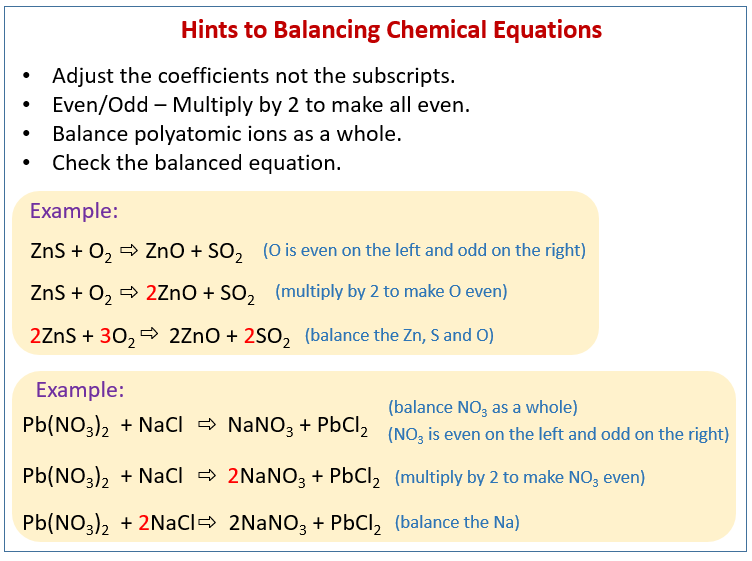
Balance chemical formulas by placing coefficients in front of them. Do not add subscripts, because this will change the formulas. Indicate the states of matter of the reactants and products. Use (g) for gaseous substances. Use (s) for solids. Use (l) for liquids. Use (aq) for species in solution in water.
Chemical Writing & Balanced Equations Upper sec Science
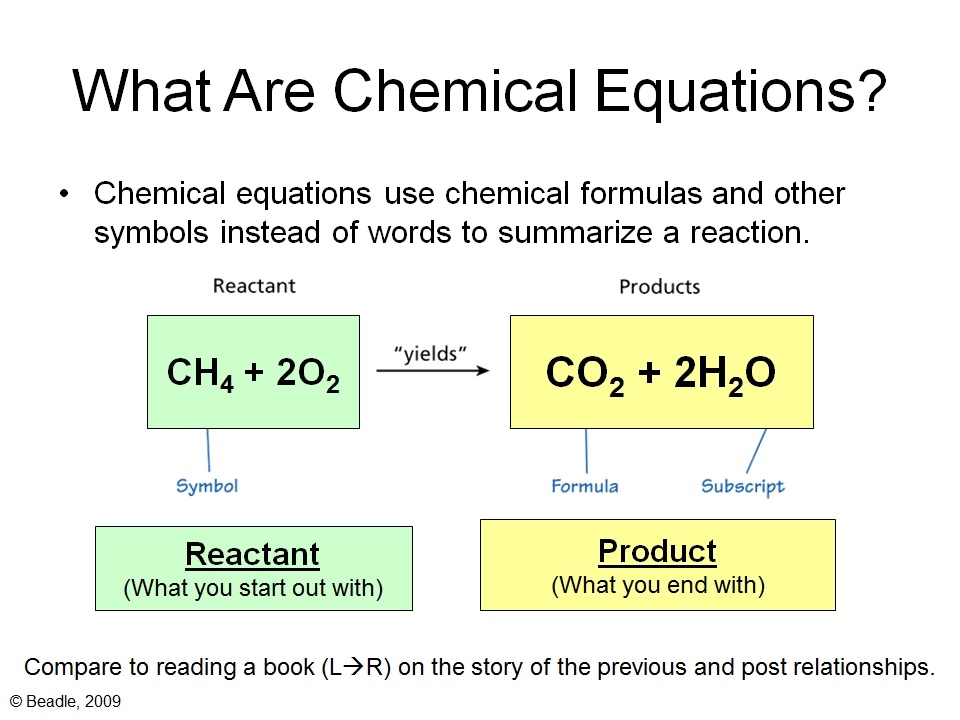
For example, let's look at the following chemical equation - Zinc + Sulphuric acid → Zinc sulfate + Hydrogen This is further simplified as - Zn + H 2 SO 4 → ZnSO 4 + H 2 Now, let us look at the number of atoms of each element on LHS and RHS - As you can see, the number of atoms of each element on LHS and RHS are the same.

50 chemical equation with name and balance ti Brainly.in
Write Down Number of Atoms. The next step for balancing the chemical equation is to determine how many atoms of each element are present on each side of the arrow: Fe + O 2 → Fe 2 O 3. To do this, keep in mind a subscript indicates the number of atoms. For example, O 2 has 2 atoms of oxygen.

50 Examples Of Balanced Chemical Equations With Answers Pdf Tessshebaylo
Here are 50 simple balanced chemical equations. 2Fe2O3+3C--->4Fe+3CO2 2)H2SO4+CaCO3--->CaSO4+H2CO3 3) 2H2+02--->2H20 4) CH3COOH+C2H5OH ---> CH3COOC2H5+H2O 5) 1 AgNO3 + 1 LiOH --> 1 AgOH + 1 LiNO3 6) CH4+2O2--->CO2+2H2O. 7) Sn+2H2SO4-->SnSO4+2H2O+SO2 8) CuO+H2SO4--->CuSO4+H2 9) Mg3N2 + 3H2O ---> 3MgO + 2NH3 10) N2 + O2 ---> 2 NO
How to balance chemical equations (solutions, examples, videos)
A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge are the same for both the reactants and the.
HelpWork balancing chemistry equations
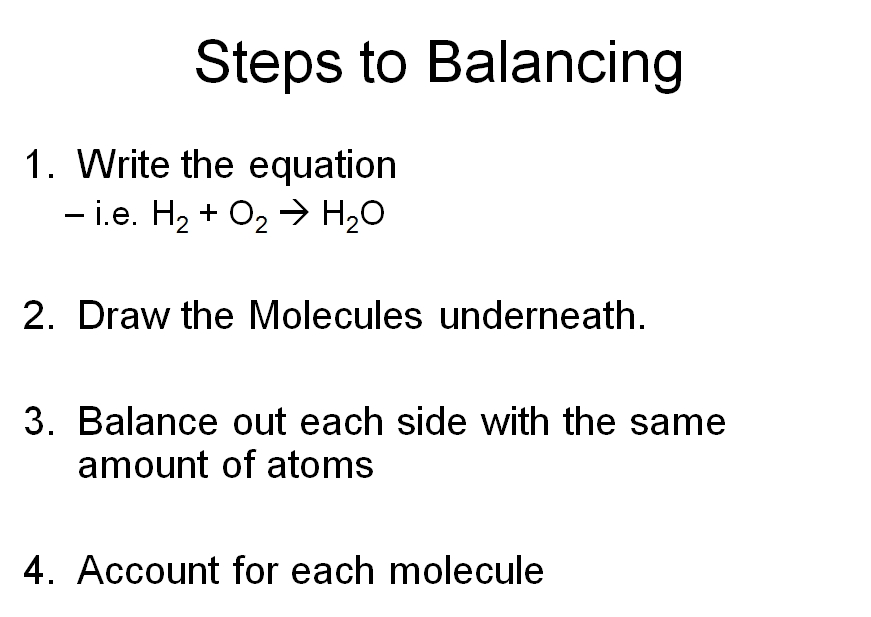
1 What is a Chemical Equation? 2 Balancing Chemical Equations Worksheets 3 Why is it Important to Balance the Chemical Equations? 4 Balancing Equations Worksheets with Answers 5 What are Different Types of Chemical Equations? 6 Balancing Equations Practice Worksheet 7 How to Balance a Chemical Equation?
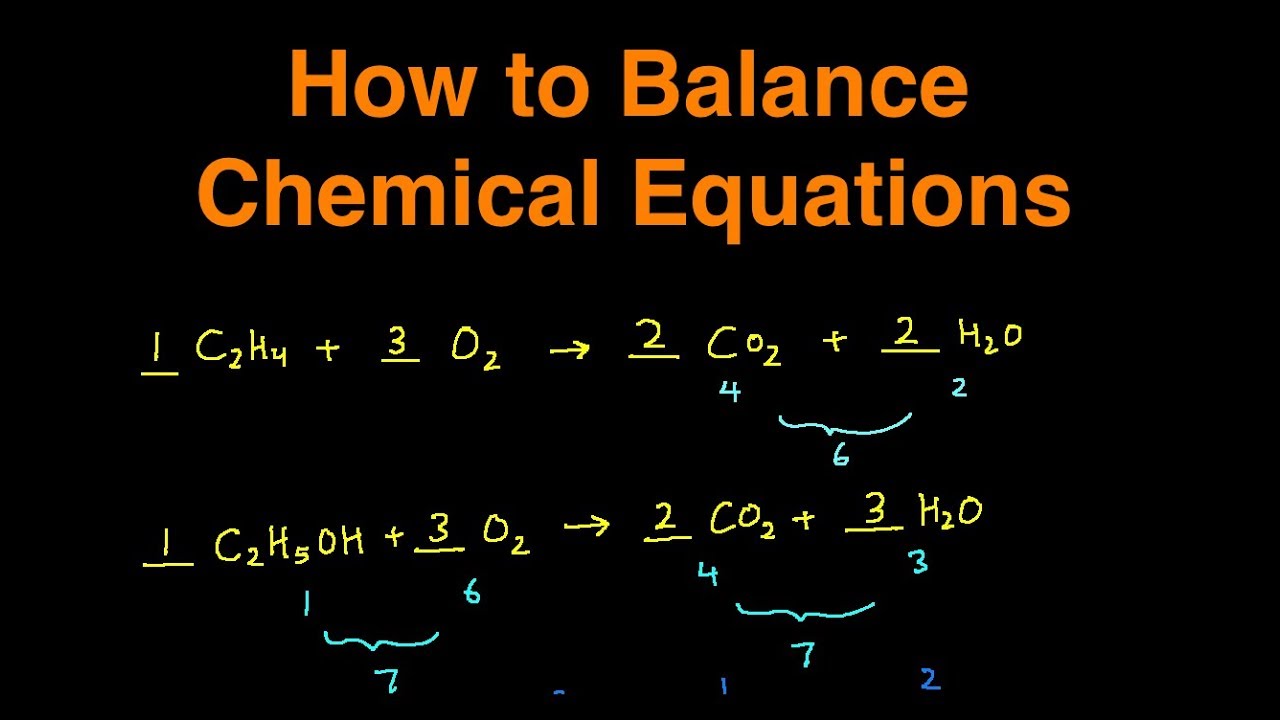
How to Balance Chemical Equations Step by Step Explanation with Examples & Practice Problems
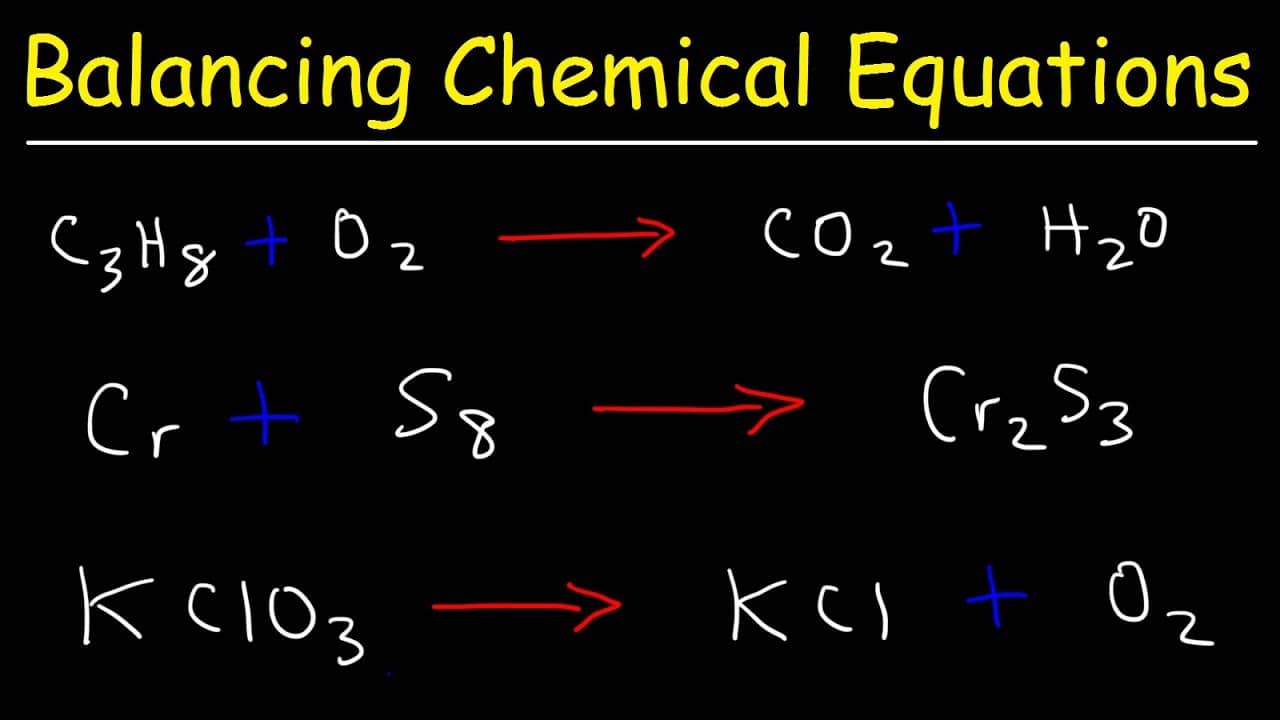
The chemical formula of propane is C 3 H 8. It burns with oxygen (O 2) to form carbon dioxide (CO 2) and water (H 2 O) The unbalanced chemical equation can be written as C3H8 + O2 → CO2 + H2O Step 2 The total number of atoms of each element on the reactant side and the product side must be compared.
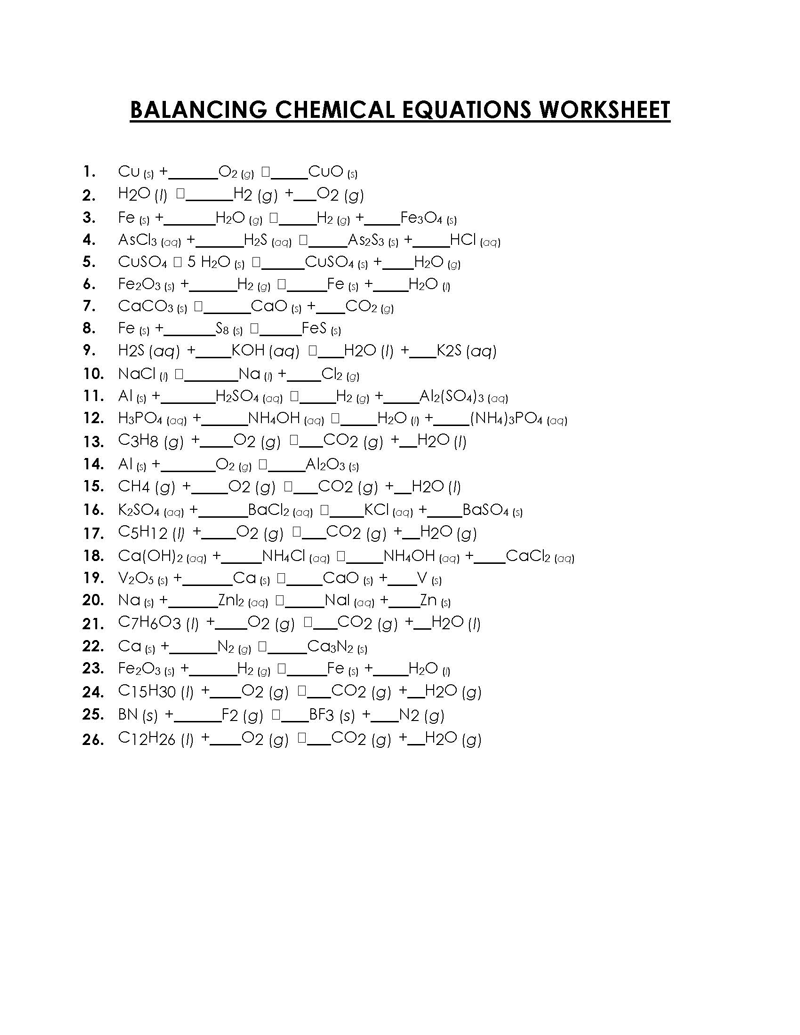
45 Free Balancing Chemical Equations Worksheets (PDF)
Chemistry Examples. Step-by-Step Examples. Chemistry. Chemical Equations and Reactions. Balance. Li + H2O → LiOH + H2 L i + H 2 O → L i O H + H 2. The law of conservation of mass states that matter cannot be created nor destroyed. There are no more or less atoms at the end of a chemical reaction than there were at the beginning.

How to Balance Chemical Equations 10 Steps (with Pictures)
1 Write down your given equation. For this example, you will use: C 3 H 8 + O 2 --> H 2 O + CO 2 This reaction occurs when propane (C 3 H 8) is burned in the presence of oxygen to produce water and carbon dioxide. 2 Write down the number of atoms per element. Do this for each side of the equation.
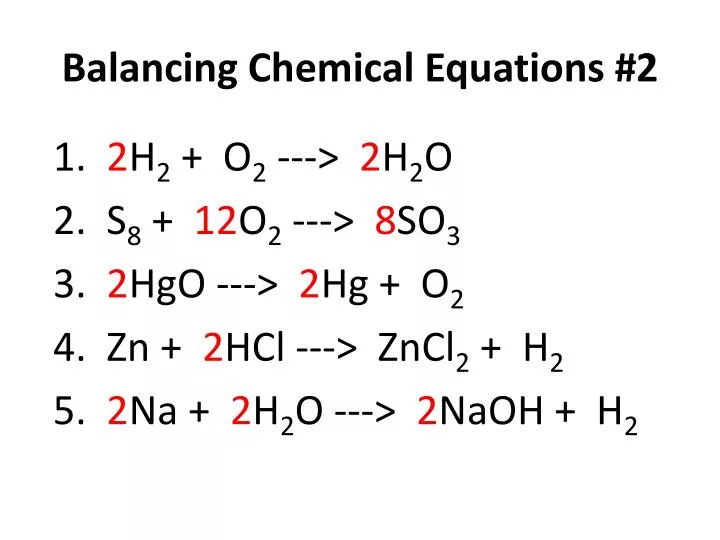
Ideal Balanced Chemical Equation Hcl And Naoh Equations Worksheet With Answers
General Chemistry Map: Chemistry - The Central Science (Brown et al.) 3: Stoichiometry- Chemical Formulas and Equations 3.1: Chemical Equations